PermanenteDocs Chat podcast on flu, COVID, and RSV vaccine safety and effectiveness updates with Sandra Fryhofer, MD, of the American Medical Association.

The most widely used test is the PCR test where a special swab is inserted into the nasal area.
Building coronavirus testing capacity
Attention turns to population testing as the nation slowly reopens
By Benjamin Seto
The Permanente Federation
If developing a vaccine is the long game in the fight against the novel coronavirus, effective testing is the short game as clinicians and public health officials seek to suppress flare-ups of infection.
With various parts of the country reopening stores and services through a phased approach, testing has been identified as an important indicator to allow for easing of stay-at-home policies and to help manage community health. Harvard University’s Global Health Institute estimated that the country needs to perform as many as 900,000 daily tests for a safer reopening of the economy; the Covid Tracking Project reported that about a third of that number is currently being performed daily.
In the early days of the COVID-19 pandemic, Kaiser Permanente recognized the importance of testing and the need to internalize operations, as outside lab vendors were overwhelmed with the wave of samples from suspected coronavirus patients. In the weeks since shelter-in-place guidelines were implemented, all Kaiser Permanente regions and their Permanente Medical Group clinical lab partners quickly set up internal testing processes, and in some cases worked diligently to expand capacity even further.
“We realized that testing is an ongoing area of interest and will become more and more important,” says Steven R. McLaren, DO, assistant medical director of Southern California laboratory care delivery systems at the Southern California Permanente Medical Group. “We made a business case to purchase large Roche analyzers that will provide us with greater throughput.”
The first of two Roche analyzers — the equipment used to process coronavirus tests — is expected to arrive at Kaiser Permanente’s Southern California laboratory by the end of this month, more than doubling the lab’s testing capacity from 2,000 to 5,000 tests a day. A second analyzer is expected by the end of summer.
New testing laboratory

In Kaiser Permanente’s Northern California region, a team of construction workers is quickly building a new 7,700-square-foot testing laboratory in Berkeley, which is expected to open in June. When up and running, the lab will be able to perform 10,000 tests a day.
“We’re using warehouse space adjacent to our current lab,” says Brian Missett, MD, an associate executive director of The Permanente Medical Group with responsibilities for the region’s laboratory operations. “We worked with capital planners and construction teams at Kaiser Permanente and the city of Berkeley to develop an aggressive timeline to open a testing facility to help our patients.”
The predominant coronavirus testing conducted at Kaiser Permanente labs is known as polymerase chain reaction or PCR, which detects the presence of RNA from SARS-CoV-2 that causes COVID-19. But other tests are being mentioned when talking about testing, which can cause confusion among patients — and even some clinicians.
The main types of coronavirus tests are:
- Polymerase chain reaction: In a PCR test, a swab is inserted into the nose to gather a small sample from the back of the throat and deep within the nose (nasopharynx). It’s highly accurate in detecting the coronavirus, and results are often available in less than 24 hours.
- Rapid molecular tests: Rapid tests detect coronavirus more quickly than the larger Roche test. Clinicians gather a simple nasopharyngeal swab and learn in a few minutes if someone is infected. Kaiser Permanente is currently using the widely available Abbott rapid tests for clinical situations when quick determination of infection is needed, such as in a hospital setting before a patient has surgery.
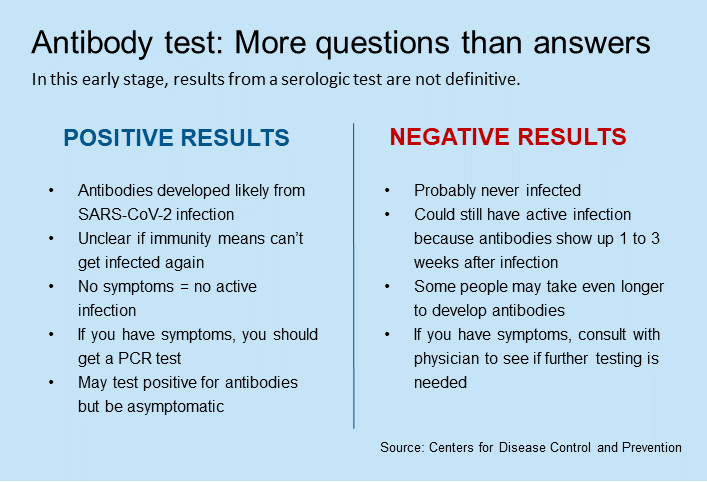
- Antibody (serologic) tests: These blood tests — which are just becoming available to doctors — identify antibodies to the coronavirus, typically 2 weeks after infection. However, so many different serology tests have hit the market without strong evidence of their validity that health care systems such as Kaiser Permanente have not begun to widely offer them.
“While serology testing is something to consider for the future, right now more studies are needed,” Dr. Missett says. Kaiser Permanente labs are actively working to obtain and validate an antibody test that will provide reliable results, and participating in research to better understand the potential role of these tests in COVID-19 management.
SCPMG’s Dr. McLaren agrees that more studies are needed on antibody tests, especially to determine whether antibodies for the coronavirus translates into immunity from future infection. However, some COVID-19 patients have been successfully treated with plasma transfusions from others who have recovered from the disease, and Dr. McLaren believes that the antibody test could be useful for identifying candidates for convalescent plasma donations.
“If we test for the antibody and it’s positive, we can create a population of patients who are potential donors of plasma,” Dr. McLaren says.
Who should get the PCR test?
With so much attention on testing, the public has also a lot of misconceptions. “The hardest question we get is when patients have had an exposure but are asymptomatic and they ask for testing,” says Mona Gahunia, DO, an infectious disease physician and associate medical director of operational excellence at the Mid-Atlantic Permanente Medical Group. “We can do the test but it’s hard to interpret what the results really mean.”
Dr. Gahunia explains that an asymptomatic patient could get a false negative test because the patient may not be “shedding” enough of the virus for the test to detect or the patient may still be in the incubation period. “Even if a test is negative in an asymptomatic patient, our recommendation for self-isolation for 14 days after a known exposure is unlikely to change,” she says.
At Kaiser Permanente, physicians use their judgement regarding who should be tested based on Centers for Disease Control and Prevention guidelines and levels of risk. The availability of testing supplies and local information on the overall spread of the coronavirus also come into play.
While serology testing is something to consider for the future, right now more studies are needed.
— Brian Missett, MD, TPMG associate executive director
For example, Kaiser Permanente’s Washington region recently expanded its testing criteria of coronavirus-related symptoms and is no longer focusing on people in high-risk groups. “We made this change recently and it did not actually increase our number of people being tested,” says Barbara Detering, MD, senior medical director of the Washington Permanente Medical Group. Dr. Detering says fewer people are calling about COVID-19 in the region, “so I suspect our expanded criteria for testing is coinciding with a decrease of illness in the community.”
Changing criteria
While testing has focused primarily on patients with symptoms, as the country reopens it will expand in phases to other at-risk populations to assist in a suppression strategy. For example, Dr. Missett says, in the future testing could include people who had contact with someone who tested positive for coronavirus. This would require increased testing capacity to meet the need.
“Once you expand to people who have had contact with someone who tested positive, that’s a massive phase,” he says. “One person with the disease, going to work or going about his or her normal day, can potentially have contact with up to 10 if not hundreds of people.”
The pandemic brings unprecedented challenges to the health care community every day. Testing capacity is often determined by the availability of testing materials. Supplies of the rapid Abbott test, for example, are limited, so hospitals must be judicious when using them.
For many Kaiser Permanente medical centers, the rapid tests are used in urgent situations such as pregnant women about to delivery and need to enter the hospital, patients requiring admission to the intensive care unit, or patients needing emergency surgery.
The Permanente physician leaders emphasize that whichever test is being considered, Kaiser Permanente is working to verify the validity and partnering with supply vendors to ensure that sufficient materials are available to conduct them.
“The real issue is testing patients who needs it most,” Dr. Missett says.

