Kaiser Permanente’s Department of Research and Evaluation in Southern California is 1 of 42 health systems selected to participate in a PCORI initiative.

HPV home-testing research supports better cervical cancer prevention and screening
Permanente physician-researcher says HPV home-testing provides convenient option to encourage regular screening
This story was updated in January 2023 to include latest statistics and responses from Beverly Green, MD.
Over the past few decades, a sea change in the prevention of and screening for cervical cancer has been taking place. An antiviral vaccine for the human papillomavirus, or HPV — the virus that causes virtually all cervical cancers — became available in 2006, and in 2018 the U.S. Preventive Services Task Force recommended primary screening for HPV as an alternative or adjunct to the standard Pap test.
Investigators with the Kaiser Permanente Washington Health Research Institute, in partnership with Permanente physician-researchers, are at the vanguard of the next transformative technology to screen and prevent cervical cancer: home-testing for HPV.

“We could eradicate cervical cancer if people got both the HPV vaccine and timely cervical cancer screening,” says Beverly Green, MD, MPH, family physician with Washington Permanente Medical Group. Dr. Green is a senior investigator with the research institute and an investigator on follow-up studies on home testing.
The HOME study (Home-based Options to Make cervical cancer screening Easy) found that mailing home-testing kits to people who were overdue for cervical cancer screening resulted in a 50% increase in screening compared with offering standard care — a pelvic exam in the doctor’s office with an HPV and/or Pap test, which finds abnormal cervical cells under a microscope.
The follow-up study is called STEP (Self-Testing Options in the Era of Primary HPV Screening for Cervical Cancer Trial). Funded by the National Cancer Institute and in collaboration with the Universities of Washington and Texas, this Kaiser Permanente study is evaluating the effectiveness of home-based HPV testing kits as they relate to improving the uptake of cervical cancer screening and to costs. Rachel Winer, PhD, of the University of Washington School of Public Health, Department of Epidemiology, was the lead scientist for the HOME and STEP studies.
Although several countries use at-home HPV tests, the U.S. Food and Drug Administration has not yet approved the kits. Research at Kaiser Permanente and other institutions is helping to pave the way for their approval in the United States.
During Cervical Cancer Awareness Month, Dr. Green discusses how research spearheaded by Kaiser Permanente could help make cervical cancer screening with accurate, easy HPV testing at home a reality in coming years.
Why do we need to make it easier for people to be screened for cervical cancer?
Pap tests have reduced cervical cancer incidence and mortality by more than 50% over the last 50 years. Nearly all cervical cancer can be prevented by identifying and removing precancers caused by high-risk HPV. However, U.S. adherence to guideline-recommended screening intervals has declined from a high of 82% in 2003 to 74% in 2016, with further declines from 2020 to 2021 due to reduced screening during the COVID-19 pandemic.
Cervical cancer screening rates vary by sociodemographic factors such as race and ethnicity, and can be influenced by well-documented barriers such as access to health insurance, knowledge about HPV and cervical cancer, beliefs about the importance of regular screening, discomfort or embarrassment with the Pap test, and lack of time and transportation to get screening appointments. People who never or rarely get screened are clear priority for outreach, as more than half of the 14,000 cervical cancers diagnosed each year occur in those who have not been recently screened.
How does the STEP study build on the HOME study?
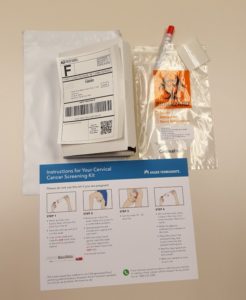
The HOME study used data from Kaiser Permanente’s electronic health record to identify people ages 30 to 64 who were overdue for cervical cancer screening and mailed them HPV self-sampling kits instead of scheduling an office visit. The STEP study reached out to 2 additional groups of people, also ages 30 to 64:
- Those who got screened regularly and were about due for their next screening
- Those with unknown cervical cancer screening status
In these 2 new groups, the STEP study compared whether educational mailing only or educational mailing with additional information on how to obtain a home test kit is a more efficient outreach strategy. Mailing everyone a kit may not be the most effective approach, particularly among those with unknown screening status because they may not need it. The study will determine which is the most effective and efficient strategy for each group and each type of strategy to motivate individuals to get screened.
What’s currently happening with the STEP study and are there any findings you can share?
The study has been completed, and we are now analyzing the data. We hope to have results to share in 2023.
Why is it important for physicians such as yourself to engage in this work?
Our team is testing whether making it easier for patients to get cervical cancer screening increases screening uptake, and secondarily help early detection of cervical cancers and precancers [abnormal cells at higher risk of becoming cancer] by taking screening out of the medical office and into patients’ homes. We found in HOME and other studies that patients prefer this convenience. Also, clinicians can have more time to focus on other things patients care about to help keep them healthy.
What have we learned from home screening for other conditions that can be applied to screening for cervical cancer?
We have performed similar studies at Kaiser Permanente in Washington to increase colorectal cancer screening. Patients due for screening were mailed fecal immunochemical tests (FIT) to complete screening at home. Colorectal cancer screening rates almost doubled, and most patients preferred completing screening in the privacy of their home.
Is there anything else about HPV home testing that you think is important for physicians and patients to understand?
Some people are worried that home HPV testing is not as accurate as getting tested in a medical facility. The home HPV kit is just as accurate as tests done in clinic and more accurate than the old tests [Pap tests]. We are lucky to now have an accurate test that patients can do in the privacy of their homes.

